Principle of electrochemical corrosion
When the hull made of iron comes into contact with the sea water, it will generate potential and electric corrosion will occur. Therefore, in order to minimize the contact between the hull and the sea water, anti-rust paint is used to isolate the hull and the sea water. However, contact with sea water due to aft shafting, thrusters or hull damage cannot be completely avoided. Therefore, a part of the ship that comes into contact with seawater will have electrochemical corrosion, and the degree of corrosion is different according to the different electrolytic conditions.
Classification of cathodic protection technologies
Cathodic protection According to the way to provide cathode current, cathodic protection is divided into two kinds of sacrificial anode method and impressed current method, the former sacrificial anode method is a more negative potential of the metal (such as magnesium, aluminum, zinc, etc.). Note: The metal is more active, more active, and more likely to lose electrons) and the protected metal structure is electrically connected, and the protection current is provided to the protected object through the continuous dissolution and consumption of the electronegative metal or alloy, so that the metal structure is protected. The latter method is to convert the external alternating current into low-voltage direct current to apply a certain DC current to the protected metal surface, so that it produces cathode polarization, when the potential of the metal is negative to a certain potential value, the anodic dissolution process of corrosion will be effectively inhibited.
The sacrificial anode and cathode protection law is generally made of zinc block alloy, and the layout has no specific requirements, as long as it is evenly distributed along the keel flow line, and the specific number is calculated according to the amount of steel (area) of the ship. Aluminum alloy can also be used, the effect is better, but it is forbidden to use in the cabin and cargo oil tank and other areas (because the potential difference is too high to cause the possibility of Mars). The general design service life of 2-3 years, the use of welding or riveting fixed on the hull shell, riveted to the later use can be easily replaced, and there are various models to choose. Where the interior area of double bottom and double wall cabin (bottom; double hull inner area) shall also be provided with sacrificial anode protection.
The following will introduce the principle and method of cathodic protection process of impressed current in detail.
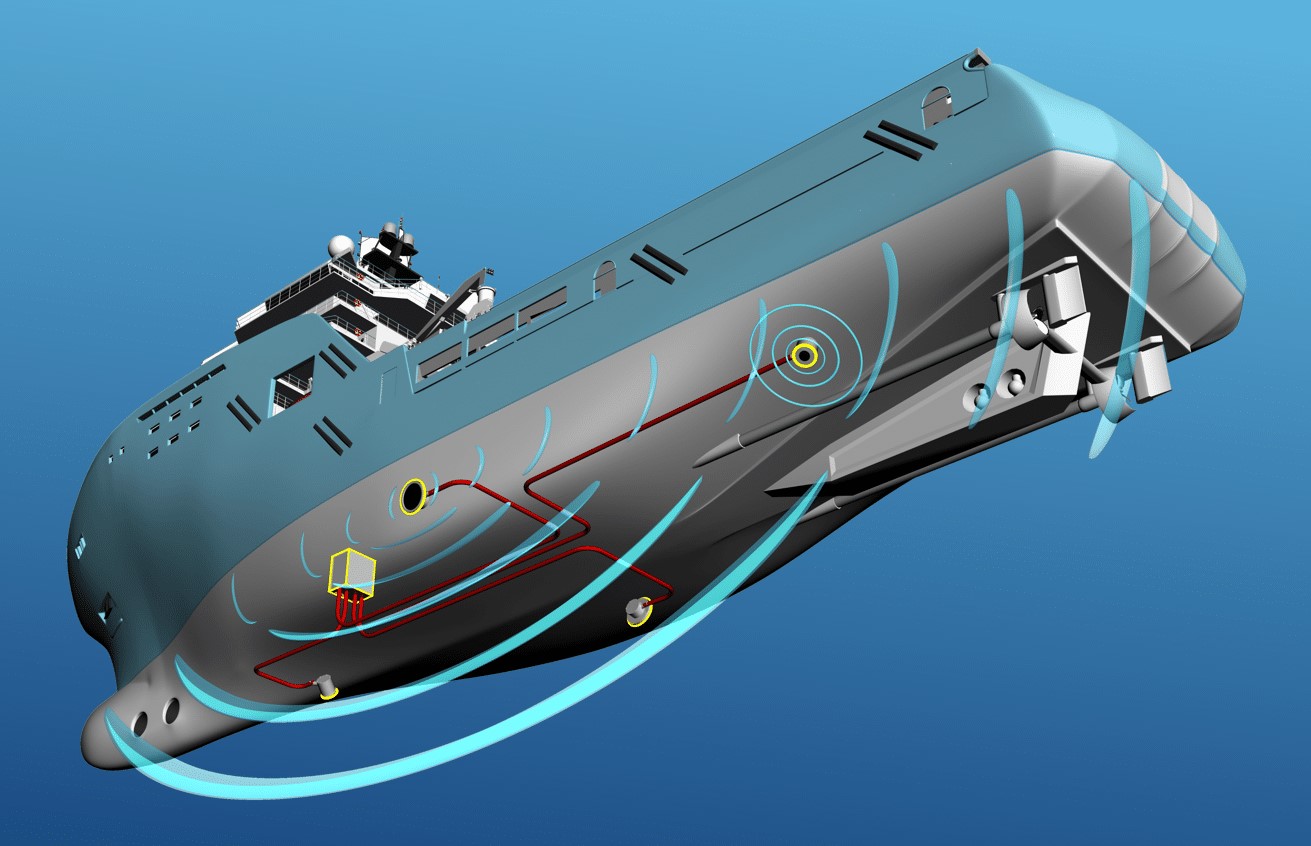
ICCP(IMPRESSED CURRENT CATHODIC PROTECTION) ICCP(impressed current cathodic protection)
The above electrochemical corrosion process is reversed: the reduction reaction takes place as the cathode, so the negative electrode of the DC power supply is directly connected, and electrons are transported to the region by the power supply, thus forming a lower potential. In the cathodic protection system of ship, the hull is used as the cathode, and the potentiostat is used to keep the hull at a low potential. This protection method uses an external DC power supply and auxiliary anode to force electrons from the soil to the protected metal, so that the potential of the protected metal structure is higher than the surrounding environment, so as to achieve the purpose of protection.
Marine impressed current cathodic protection system consists of the following parts :① DC power supply, ② auxiliary anode, ③ reference electrode. In addition, in order to make the protection current of the anode output more uniform and avoid over-protection of the structure near the anode, the anode shielding layer must sometimes be painted around the anode. The combined application of cathodic protection and coating enables the ship to obtain the most economical and effective protection. A good coating can protect more than 99% of the outer surface of the hull from corrosion. The cost of cathodic protection usually only accounts for 1% to 5% of the cost of the hull, and the service life of the ship hull can be doubled or even tens of times longer, therefore, this technology has been widely recognized by people, and has been more and more widely used in the fields of ships, port facilities, Marine engineering, petrochemical, electric power, municipal and other fields, and the prospect is very broad.
The method of impressed current is not only suitable for metal structures, but also for concrete structures, and is the only effective anti-corrosion measure for concrete reinforcement so far. This protection method is suitable for a variety of scenarios, including but not limited to:
1, the protection of the underwater metal part of the ship: sea water is a strong corrosive medium, sailing in which the ship will suffer different degrees of corrosion, seriously affecting the navy’s combat ability, therefore, the ship’s corrosion prevention problem by the attention of various countries. Since the British chemist Davy first applied cathodic protection technology in 1824, after more than 190 years of research, cathodic protection technology has been greatly developed. The impressed current cathodic protection device installed on the hull constantly transmits electrons to the hull and the bottom of the ship to provide anti-corrosion protection for the metal parts below the ship that are soaked in seawater. With the impressed current cathodic protection, it effectively prevents electrochemical corrosion in the flooded area and more firmly escorts the ship. The entire underwater area of the ship will become a cathode, which will become alkaline due to excessive hydrogen and oxygen ions. Therefore, the coating used in the ship bottom area must have good water resistance, alkali resistance, wear resistance, and the outer coating should also have the anti-fouling property to prevent the attachment of Marine organisms.
2, corrosion protection of steel bars of cross-sea bridge : impressed current cathodic protection is an advanced protection method for corrosion protection of steel bars of cross-sea bridge. Titanium mesh, which is more active than steel bars, is set between steel bars and concrete surfaces as the anode and steel bars as the cathode, and current is applied to the surface of the protective cladding structure to protect the steel bars from corrosion and make the structure meet the design life requirements .
3, protection of the oil-water separator : impressed current cathode protection law is also used to protect the service life of the oil-water separator . It can effectively reduce the risk of perforation leakage due to corrosion of the separator, thereby reducing environmental pollution, reducing the number of major repairs, and extending the service life of the separator, thus producing obvious economic and social benefits.
4, cathodic protection of large equipment : for the equipment in the environment with high soil resistivity, such as long distance oil and gas transmission buried underground industrial pipelines and large storage tank groups of oil and other industrial raw materials, the cathodic protection method of impressed current .
FROM BLUEWAV TECHNOLOGY CO.,LTD