Salicylic acid is a pharmaceutical intermediate that can be used as a synthetic aspirin. The annual production and use of salicylic acid is very large, and it is discharged into the water body in a variety of ways, and it is a high content of PPCPs pollutants in natural water bodies. According to the relevant literature in 2008, the salicylic acid pollutants in the water system of Dongjiang River in China can reach 1μg·L-1, and the salicylic acid has been detected in the water environment of the Pearl River Delta region! Concentrations up to 2μg·L-1. Chemical oxidation is an important method to degrade organic pollutants in sewage treatment. Sodium hypochlorite as a strong oxidizing agent, oxidation performance is very good can make organic matter fade, can destroy bacteria and algae cell membrane enzyme system to inactivate it, with oxidation, sterilization, disinfection, fading effect, while sodium hypochlorite solution has no free molecular chlorine, can reduce trichloromethane and other disinfection byproducts. Sodium hypochlorite and salicylic acid will oxidize, which has a good removal effect on salicylic acid. BLUEWAV’s on-site preparation of sodium hypochlorite solution generators reduces time and freight hassle-plug and play.
Salicylic acid is the main raw material of salicylic acid drugs and an essential ingredient in many medicines and cosmetics. It exists in large quantities in the environment and may cause damage to the ecosystem. Salicylic acid and its derivatives have a wide range of applications. In the pharmaceutical industry, salicylic acid can be used as a pharmaceutical intermediate to synthesize aspirin, and salicylic acid can also be used as a preservative. Therefore, in this experiment, sodium hypochlorite was selected as an oxidizing agent to study the influencing factors, degradation conditions and changes in product toxicity during the oxidative degradation of salicylic acid solution by sodium hypochlorite. The influence of different pH value and temperature on the reaction process was mainly studied, and the degree of reaction in the degradation process and the toxicity of the degradation products were analyzed, with a view to preliminarily establishing the technical method of sodium hypochlorite treatment of salicylic acid and providing scientific basis for future research.

Experimental materials and methods
Reagent: salicylic acid, purity >98%; Methanol, chromatographically pure; Sodium hypochlorite, ammonium acetate, potassium dihydrogen phosphate, disodium hydrogen phosphate dodecahydrate, sodium chloride and other drugs are analytically pure. Luminous luminous Bacillus freeze-dried powder; The water used in the experiment was ultra-pure water.
Instrument: AX423ZH electronic balance, UP400HE ultrasonic cleaner, Milli-QReference pure water system, Waters1525-2747 High performance Liquid Chromatograph, Chromatographic column C18(5μm, 4.6x150mm), BL200S collector constant temperature heating magnetic Agitator, DXY-2 biological toxicity detector, PHS-3C precision pH meter.
Test method
Weigh 50mg salicylic acid and prepare 50mg·L-1 salicylic acid reserve solution. The experiment was carried out at room temperature of about 25℃. 50mL buffer solution with a set pH value was put into a brown reagent reaction bottle with a volume of 80mL, and then 100μL salicylic acid of different concentrations was added to the reaction bottle, and the magnetic stirrer was used to stir. Quickly fill the reaction bottle with 50μL sodium hypochlorite solution and start timing, sampling at different times. The residual chlorine in the sample was quenched by Na2S203(0.1mol.L-1) and the concentration of salicylic acid was determined by high performance liquid chromatography (HPLC).
Determination method
The salicylic acid content was analyzed by high performance liquid chromatography. The instrument used is a high performance liquid chromatograph with a fluorescence detector. Through various tests, the optimal determination conditions of salicylic acid were determined as follows: separation column C18(5 um,4.6x150mm), mobile phase :0.02mol.L-1 ammonium acetate aqueous solution: pure methanol =4:1(volume ratio) mixed solution, flow rate of 1 mL·min-1, sample size of 20μL, column box temperature of 30 ℃. The fluorescence detector has an excitation wavelength of 300 nm and an emission wavelength of 407nm. Figure 2-1 shows the high performance liquid chromatography of salicylic acid aqueous solution with a concentration of 0.1mg.L-1.
Toxicity test method
According to the national standard method GB/T1S441-1995, the change of toxicity of sodium hypochlorite during the oxidation of salicylic acid aqueous solution was tested by using the lyophilized powder of T3 small species of luminous Bacillus. Use a syringe to pour 1mL of 2.5% sodium chloride solution into a freeze-dried powder ampere bottle and mix well. After 2min, the luminescence of luminescent bacillus began to recover, and the luminescence intensity of luminescent bacillus tended to stabilize after about half an hour. At this point, the luminobacter toxicity test experiment can be started. The relative inhibition rate of the reaction solution to luminescence of luminous bacillus during the degradation of salicylic acid was measured to indicate the change of the compound toxicity of the parent and the degradation product during the oxidation of salicylic acid by sodium hypochlorite. The higher the inhibition rate, the higher the toxicity, and vice versa. It is less toxic. Each sample should be tested three times in parallel, and a blank sample should also be set up for control. The relative inhibition rate (%) of luminous Bacillus was:
I%=(1-I1/I0)x100%
In the formula, I1 is the luminosity of the sample and I0 is the luminosity of the control.
The toxicity study of the reaction process needs to remove the influence of sodium hypochlorite alone on the relative inhibition rate of luminescence intensity of luminous Bacillus in the process of being consumed by the quenching agent sodium thiosulfate. The final relative inhibition rate (I% water) is as follows:
I% water =I%-I% times
In the formula, I% water is the relative inhibition rate of the final degradation process of salicylic acid, and I% times is the relative inhibition rate of the consumption process of sodium hypochlorite by the quencher.
Experimental results and analysis
According to the test method, the reaction temperature was controlled to be 25℃, the pH value was 7, and the reaction time was controlled to be 30 minutes, and the relationship between the concentration of salicylic acid and time was measured respectively under the condition that no sodium hypochlorite was added. After the addition of sodium hypochlorite, the reaction rate constant of salicylic acid is obviously not zero. With the passage of reaction time, the concentration of salicylic acid becomes smaller and the degradation rate becomes higher and higher. For salicylic acid solution without sodium hypochlorite, the concentration of salicylic acid does not change with the change of time, that is, the effect of salicylic acid hydrolysis on the system can be ignored within the experimental time range.
Under normal circumstances, the influence of the concentration of the reaction solution on its reaction rate is not as significant as that of the temperature. According to Van’t Hof rule, in the general reaction, the reaction rate will continue to increase with the increase of temperature, and the reaction rate can be changed by 2 to 4 times for every 10℃ change in temperature. When c(SA) was 0.1mg·L-1, c(available chlorine) was 30 mg·L-1· and pH value was 7.0, the influence of temperature on the oxidation of salicylic acid by sodium hypsosinate was investigated. The following figure shows the relevant data of the influence of temperature on the reaction.
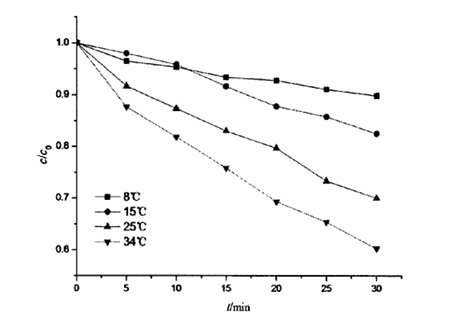
It can be seen from the figure that the reaction rate constant of salicylic acid at different reaction temperatures has obvious differences. With the increase of temperature, the value of k increases correspondingly, and its law is more in line with van’t Hoff’s rule.
Study on the toxicity of sodium hypochlorite for oxidative degradation of salicylic acid
When a reaction continues, the toxicity of the whole system will change, on the one hand, because of the formation of new reaction products with unknown toxicity, on the other hand, because of the continuous reduction of the original compound. The oxidation reaction can destroy the original organic compounds and reduce their content, but new products are generated during the reaction process, and the influence of these intermediate products on the toxicity of the whole reaction system is uncertain. Therefore, in this experiment, the toxicity determination method of luminous Bacillus was used to detect the changes of the combined toxicity of the parent compound and its products, and the luminous inhibition rate was used to indicate the magnitude of toxicity.
Under the conditions of pH 7, temperature 25℃, initial concentration of salicylic acid was 0.1mg.L-1, and effective chlorine concentration of sodium hypochlorite was 30mg·L-1, the reaction was carried out for 5 hours, and the relative inhibition rate was calculated by sampling at different time points to determine the changes in toxicity of salicylic acid aqueous solution in the process of disinfection and oxidation with sodium hyponitine.
The results showed that the relative luminescence inhibition rate (I%) of pure salicylic acid solution against luminous Bacillus was 28.4%. The relative luminescence inhibition rate began to change after the addition of sodium hypsosinate to start the reaction. In the first half hour, the relative luminescence inhibition rate of degraded samples first decreased, then increased, and then remained relatively stable. Overall, the toxicity was less than that of salicylic acid solution before the reaction began. The results showed that the toxicity of salicylic acid reaction system was first decreased, then increased, and then decreased, and the toxicity of the product was not significantly greater than that of salicylic acid. It may be because in the early stage of the reaction, due to the small amount of products generated, the reaction is mainly based on the oxidative degradation of salicylic acid, so the relative luminescence inhibition rate is smaller. As the reaction progresses, the amount of intermediate products produced may accumulate, increase, or a highly toxic product is produced, at which time the relative luminescence inhibition rate shown increases. With the passage of the reaction process, these intermediates are also oxidized by sodium hypochlorite, so the relative inhibition rate of luminescence is reduced. In the middle and late stage of the reaction, the relative luminescence inhibition first increased to 15.5% and then decreased to 5.8%, indicating that the overall toxicity increased first and then decreased. It can be inferred from the results that while salicylic acid is continuously oxidized and degraded, some intermediate products with certain toxicity are produced, and the priority of oxidative degradation of products by sodium hypochlorite is lower than that of salicylic acid, so the generated intermediate products continue to accumulate and the relative luminosity of the sample is relatively low. With the further deepening of the reaction, these products are also removed by sodium hypochlorite, so the relative luminescence inhibition rate of the final reaction liquid is reduced. In general, the whole reaction system will produce some toxic products, indicating that there may be some risks in the process of sodium hypochlorite oxidation of salicylic acid, which can be further studied.
In this experiment, salicylic acid was selected as the target of degradation, and the oxidation removal effect of sodium hypochlorite was studied by high performance liquid chromatography (HPLC) according to its physicochemical properties. The following conclusions are obtained through the experiment:
(1) Sodium hypochlorite can be oxidized to remove salicylic acid. The initial concentration method was used to fix the concentrations of sodium hypochlorite and salicylic acid respectively. Under the condition of pH-7 and temperature =25℃, the oxidative degradation of salicylic acid by sodium hypochlorite was a first-order reaction to salicylic acid and sodium hypochlorite, that is, the oxidative degradation of salicylic acid by sodium hypochlorite was in line with the characteristics of second-order kinetic reaction. Increasing the concentration of sodium hypochlorite can accelerate the reaction. The initial concentration of salicylic acid was negatively correlated with the apparent reaction rate constant.
(2)pH value has an important effect on the reaction of sodium hypochlorite oxidizing salicylic acid. Under the combined action of the ionization of salicylic acid and the stability of sodium hypochlorite at different pH values, the reaction is faster when the solution is acidic, which is conducive to the degradation of salicylic acid.
(3) Temperature has a relatively large effect on the reaction of sodium hypochlorite oxidation of salicylic acid, which conforms to van’t Hoff’s rule. The apparent activation energy Ea=43.22 KJ.mol-1 of sodium hypochlorite oxidative degradation of salicylic acid, and the change of temperature can cause the change of reaction rate constant k in the positive direction, so the reaction at higher temperature is more conducive to the removal of salicylic acid.
(4) The toxicity changes of sodium hypochlorite during the oxidative degradation of salicylic acid were measured by luminobacter toxicity test. The results showed that in the process of oxidative degradation of salicylic acid, the toxicity of the sample solution was not stable, and the relative inhibition rate fluctuated significantly. In the middle, there were two rising and falling processes. At the end of the reaction, the toxicity of the whole reaction system became smaller.
BLUEWAV sodium hypochlorite generator secondary purification salt quality, purity only 89%, with many years of export experience, a variety of models to meet your production needs, in good faith to serve you.
Bluewav Technology Co.,Ltd.
Whatsapp: +86 18610563976 | Website :https://www.bluewaVv.com
This article is reproduced from a paper on the oxidative degradation of salicylic acid by sodium hypochlorite by Feng Jiahao from Henan Normal University, China

