Currently, electrolytic brine sodium hypochlorite generators are increasingly used in water treatment and disinfection due to their safety, simple and readily available raw materials, and low operating costs. We know that when electrolytic brine generates sodium hypochlorite solution, it also brings hydrogen as a by-product, which contributes to environmental safety. It brings risks. Proper treatment and emission of hydrogen meets national safety requirements and is a very ideal disinfection product. Producing 1kg of effective chlorine produces 0.35m³ of hydrogen. The greater the output of the equipment, the greater the hydrogen production. A large amount of hydrogen is diluted and released into the atmosphere to waste. Lose.
Chlorination system process principle:
The sodium hypochlorite generator is a new type of equipment used to produce sodium hypochlorite solution on site. It is suitable for various occasions that require chlorine-containing disinfection.
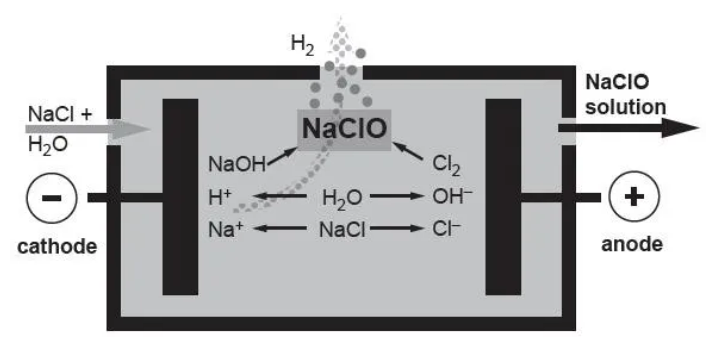
The electrolysis process of the electrolyzed brine sodium hypochlorite generator is an electrochemical reaction process. The raw materials are: salt + electricity + water, and the sodium hypochlorite solution produced is of pure quality. Its working principle is: under the action of a certain cell voltage, the sodium chloride solution undergoes a series of electrochemical reactions in the electrolytic cell, eventually generating a sodium hypochlorite solution.
Reactions in electrolytic cells:
(1) 2NaCl + 2H2O = 2NaOH + Cl2 + H2
² Anodic reaction: 2NaCl => 2Na+ + Cl2 + 2e-
² Cathode reaction: 2H2O + 2e- => H2 + 2OH-
The generated chlorine gas reacts with the generated sodium hydroxide solution to generate sodium hypochlorite solution.
(2) Cl2+ 2NaOH = NaCl + NaClO + H2O
² Interpolar reaction: 2NaOH + Cl2 =>NaClO + NaCl + H2O
The above two equations lead to: the main reaction of sodium hypochlorite generator electrolysis:
(3) NaCL+ H2O = NaCLO + H2↑
² Total reaction: NaCl + H2O =>NaClO + H2
The process of generating sodium hypochlorite also produces a considerable amount of hydrogen. According to this calculation, approximately 0.35 liters of H2 are generated for every 1 gram of NaCLO produced.
In the future, the acquisition of hydrogen collected will be a good thing for the country and the people; it can not only obtain cheap disinfection products for water disinfection, but also gain profits from selling hydrogen, which will bring benefits to users of sodium hypochlorite generators.